Anti-fungal Drug Design and Delivery
Designing anti-fungal peptides
Recently, the development of antifungal drug has become an important topic because the risk of fungal infection has substantially increased in immunocompromised patients. Antimicrobial peptides (AMPs) are very attractive as antifungal drug candidates because their membrane disruption mechanism through carpet or toroidal pores suggests low drug resistant development. The 14-helical β-peptides are actively studied in various fields such as the regulation of protein-protein interactions, antimicrobial peptides, cell penetrating peptides, self-assembled helix-bundle quaternary structure, and liquid crystal.
We studied structure-activity relationship of antifungal 14-helical β-peptides. We showed that both antifungal activity and hemolysis are strongly correlated with hydrophobicity. Additionally, we showed that helical structure-forming propensity impacts on the window of selective antifungal activity, with more stable structures eliciting specificity for C. albicans over a wider hydrophobicity range. As a result, we provided a well established platform to analyze the structure activity relationship based on hydrophobicity. We also provided insight into how hydrophobicity and helicity govern specific antifungal activity of 14-helical β-peptides.
This work is a collaboration with the laboratory of David Lynn.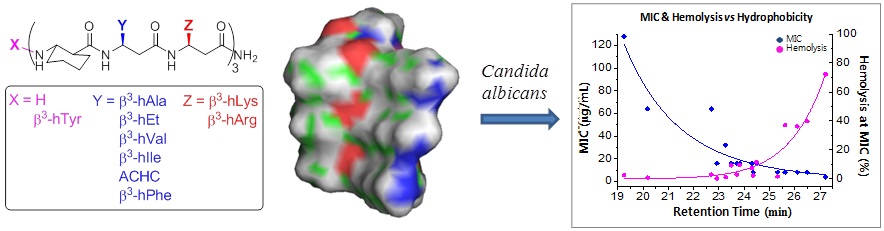
Figure modified from Lee, Myung-Ryul, et al. "Hydrophobicity and Helicity Regulate the Antifungal Activity of 14-Helical β-Peptides." ACS chemical biology 9.7 (2014): 1613-1621.
Designing drug delivery devices
Biofilms associated with implanted medical devices are a major source of hospital-acquired infections. Candida albicans causes the highest number of systemic fungal infections with 30-60 % mortality rates. We have developed a materials-based approach to prevent C. albicans biofilm formation on catheter tube segments by incorporating potent antifungal β-peptides in polymer-based polyelectrolyte multilayer (PEM) coatings. β-peptides are stable synthetic mimics of antimicrobial β-peptides and are considered less likely to lead to acquired resistance by pathogens!
We used a fill-and-purge approach to fabricate multilayers of poly-L-glutamic acid (PGA) and poly-L-lysine (PLL) on the inner surfaces of polyethylene catheters and loaded them post-fabrication with an antifungal β-peptide. This approach leads to a gradual release of active β-peptide over a period of ~120 days. PEM-coated catheters loaded with β-peptide exhibited antifungal activity for short and longer durations against planktonic C. albicans cells incubated intraluminally and also reduced biofilm formation on the inner surfaces of the catheters by ~80 %. Overall, our results establish that PEMs loaded with β-peptides are antifungal and effective in preventing fungal biofilm formation and demonstrate the potential of this approach to prevent medical device-associated fungal infections.
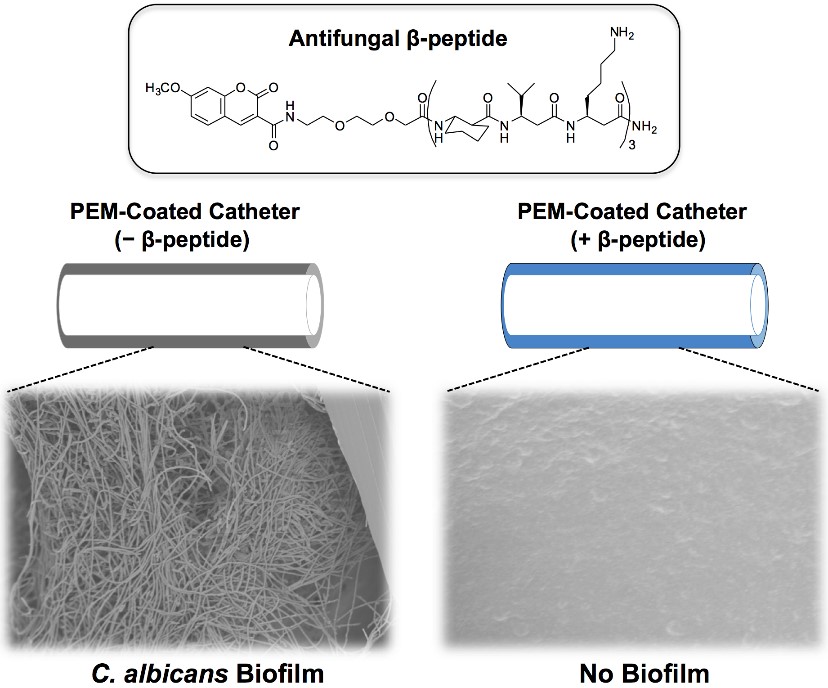
Figure modified from Raman, Namrata, et al. "Polymer multilayers loaded with antifungal β-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes." Journal of Controlled Release 191 (2014): 54-62.